Resources
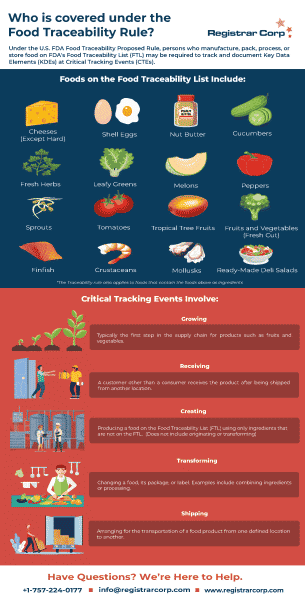
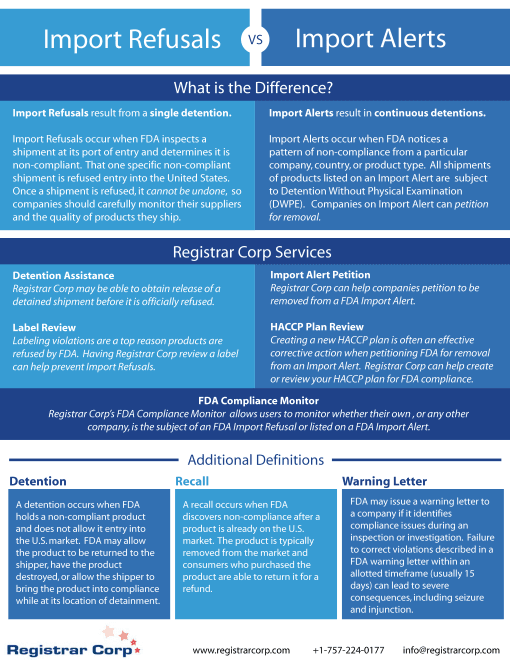
Infographics
Useful Reading

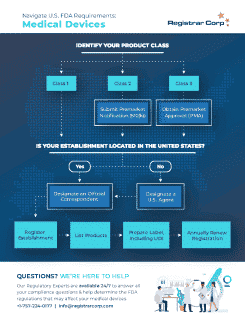
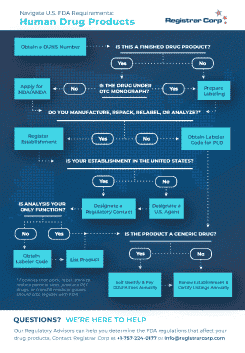
How to Get FDA Approval
It is a common misconception that all FDA-regulated products require FDA approval before being marketed in the United States. Registrar Corp created this guide to help you determine if your product requires FDA approval.

FDA and USDA – Who Regulates What
It can be difficult to determine whether a meat or poultry product is regulated by FDA or USDA. Registrar Corp created a guide to help clarify the difference between FDA and USDA jurisdiction.

FDA and NAFTA
View detailed information on U.S. Food And Drug Administration (U.S. FDA) and its laws and standards.
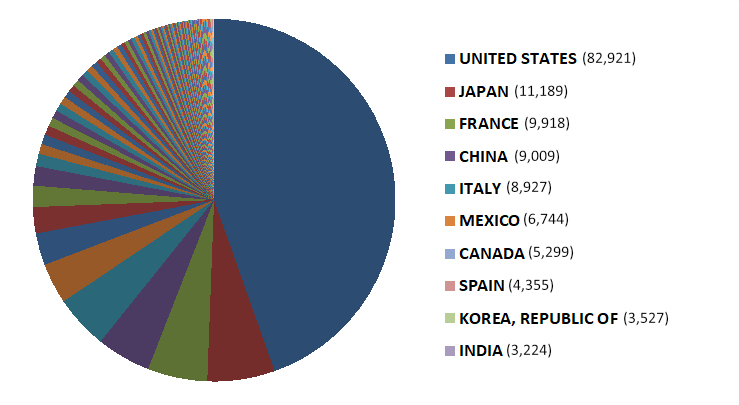
2019 Food Facility Registration Data
View the number of food facilities registered with FDA by country.

How do I get an FDA Certificate of Registration?
View the number of food facilities registered with FDA by country.
Videos
Assistance with U.S. FDA Regulations
U.S. FDA Food Facility Registration
FDA Compliance Monitor
Facility 360
U.S. FDA FCE-SID Regulations
U.S. FDA Food Labeling Regulations
U.S. FDA Unique Device Identifier (UDI)
Free Tools
FDA Compliance Monitor Savings Calculator
Registrar Corp’s FDA Compliance Monitor saves you thousands of dollars on your supplier monitoring process. See how much today.
Get StartedCheck your RegiScore
U.S. Importers use RegiScore to gauge the risk of buying from your company. Check your RegiScore for free.
Get StartedFood Safety Wizard
Registrar Corp’s Food Safety Wizard provides food facilities guidance regarding the FDA food safety requirements for their specific food products.
Get StartedQualified Facility Attestation Wizard
Registrar Corp’s Qualified Facility Wizard can help determine whether your facility is eligible to file a Qualified Facility Attestation.
Get StartedFCE Wizard
Registrar Corp’s FCE Wizard is a free tool that allows users to identify whether their products are subject to FCE and SID regulations.
Get StartedSID Verifier
Registrar Corp’s SID Verifier is a free tool that allows users to verify whether SIDs are currently on file in FDA’s database.
Get StartedAdditional Tools
Prior Notice Express
Registrar Corp’s Prior Notice Express makes filing prior notice quick and easy and decreases potential filing errors.
Get StartedFDA Compliance Monitor
Registrar Corp’s FDA Compliance Monitor® allows users to easily monitor the compliance status and history of any FDA-regulated company.
Get StartedFacility 360
Track your compliance, gauge your risk to U.S. Importers, and monitor your shipments all in one simple, secure platform.
Get StartedShipment Monitoring
Protect Your Company From Unauthorized Shipments. Monitor shipments associated with your FDA Registration Number.
Get StartedWebinars
-

Compliance Requirements for U S Food and Drug Administration FDA by Registrar Corp
-

U.S. FDA Regulations for Animal Feed
-

Six Factors that Affect Your Shipments to the U.S.
-

How to Prepare for an FDA Inspection
-

PCQI 101: How to Become a Preventive Controls Qualified Individual and Why
-

U.S. FDA Foreign Supplier Verification Program (FSVP) Requirements
-

U.S. FDA Preventive Controls Requirements
-

U.S. FDA Food Labeling Rules - The New Normal
-

3 Ways to Protect Your Brand with Facility 360
-

PCQI 101: How to Become a Preventive Controls Qualified Individual and Why
-

GFSI Certification 101
-

FDA Registration and U.S. Labeling Requirements for Winemakers