How to Get FDA Approval
How to get FDA approval depends on the type of product you are marketing in the United States. FDA does not require FDA approval for all types of products. Read below to learn what products require FDA approval and how to obtain it when necessary.
FDA Approval of Food, Beverages, and Dietary Supplements
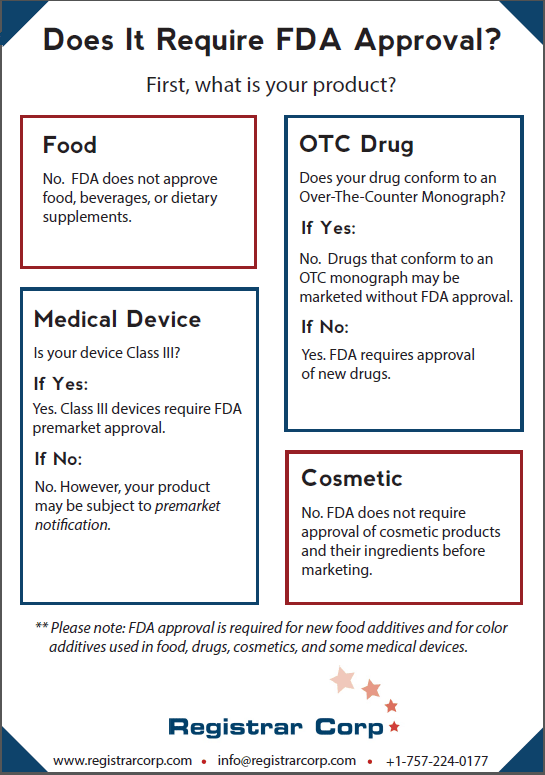
FDA does not approve food, beverages, or dietary supplements. Food facilities do not have to obtain any type of certification or approval before distributing products in the United States. Food facilities are required to register with FDA, but being registered with FDA does not indicate FDA approval of the facility or its products.
New food additives do require FDA approval. If a manufacturer wishes to use a new food additive in his product, he will need to put the additive through appropriate testing and prove to FDA that the additive is safe.
FDA Approval of Drug Products
Whether or not a new drug product requires FDA approval depends on whether the new drug conforms to an over-the-counter (OTC) monograph. OTC monographs establish conditions under which FDA has pre-determined a drug will be safe and effective. Once an OTC monograph is final, drug establishments can market OTC drugs that conform to the monograph without FDA approval. FDA also uses enforcement discretion to allow certain drugs to be marketed without approval if they conform to tentative final monographs.
If a new drug does not comply with a monograph, it will require FDA approval. To get FDA approval, drug manufacturers must conduct lab, animal, and human clinical testing and submit their data to FDA. FDA will then review the data and may approve the drug if the agency determines that the benefits of the drug outweigh the risks for the intended use. Marketing a new drug that does not conform to an OTC monograph without FDA approval is considered as marketing an unapproved new drug, which is a prohibited act under the Food, Drug, and Cosmetic Act (FD&C Act).
Though FDA approves new drugs, the agency does not approve compounded drugs. Drug establishments must register with FDA and list their products, but neither registration nor listing indicates FDA approval of the establishment or its products.
FDA Approval of Medical Devices
FDA places medical devices into one of three risk-based categories: Class I, Class II, and Class III. Class III devices are the highest-risk devices and the only devices that require FDA premarket approval. Manufacturers of Class III devices must demonstrate to FDA that the device provides reasonable assurance of safety and effectiveness.
Class I and II devices do not require FDA approval. These devices, unless exempt under the FD&C Act, must submit premarket notification (510(k)) to FDA instead. The purpose of a 510(k) is to demonstrate to FDA that the device is substantially equivalent (as safe and effective) to an already legally marketed device. If FDA determines that the device is indeed substantially equivalent to a legally marketed device, the agency clears the product for marketing rather than approving it.
Device establishments must register with FDA and list their devices, but neither registration nor listing indicates FDA approval of the establishment or its devices.
FDA Approval of Cosmetics
FDA does not require approval of cosmetic products and their ingredients (other than color additives) before marketing. Cosmetic companies are not required to register with FDA, but cosmetics do have to be safe for their intended use.
It’s important to be aware that certain claims made in cosmetic labeling can cause FDA to regulate a cosmetic product as a drug. In some cases, this could cause the product to require FDA approval.
FDA Approval of Color Additives
FDA approval is required for color additives used in food, drugs, cosmetics, and some medical devices. Certain high-risk colors also require FDA color batch certification of every individual batch. Color additives may only be used in compliance with their approved uses, specifications, and restrictions. Products that contain unapproved color additives are considered to be adulterated under the FD&C Act.
Labeling FDA Approved Products
Manufacturers of drugs and devices that do require FDA approval may include the phrase “FDA Approved” on the product’s labeling, as long as the manufacturer has received a letter from FDA confirming its approval. The FDA logo should not be used on a product’s labeling whether the product is approved or not. Use of the FDA logo could imply that the product is endorsed by FDA, therefore unauthorized use of the logo may violate federal law. Manufacturers who use FDA’s logo on their product labeling may be subject to civil or criminal liability.
Whether their products require FDA approval or not, food facilities, drug establishments, device establishments, and cosmetic companies must adhere to FDA’s Current Good Manufacturing Practices (CGMPs) and extensive labeling requirements. For those products that do require approval, such as certain drugs and devices, labeling is approved when the product is approved. However, labels are not generally subject to FDA approval.
You may be wondering how FDA enforces its requirements when so many products do not require premarket approval. FDA enforces its requirements through routine facility inspections and randomized shipment inspections at the U.S. border.
Registrar Corp helps food, drug, medical device, and cosmetic companies comply with U.S. FDA regulations. Registrar Corp can register a company with FDA, list its products with FDA, and review product labels for FDA compliance. Registrar Corp can also help submit color additives to FDA for color batch certification.
For additional questions regarding how to get FDA approval and other FDA regulations, or to learn more about Registrar Corp’s services, contact +1-757-224-0177 or chat with a Regulatory Advisor 24 hours a day at regstaging.wpengine.com/LiveHelp.

