Is Your Food Label Ready?
Businesses that average over $10 million in annual sales must update their food labels for compliance with new U.S. Food and Drug Administration (FDA) labeling rules by January 1, 2020.
FDA’s new rules mandate significant changes to the Nutrition Facts Chart format, serving sizes, daily values, and more.
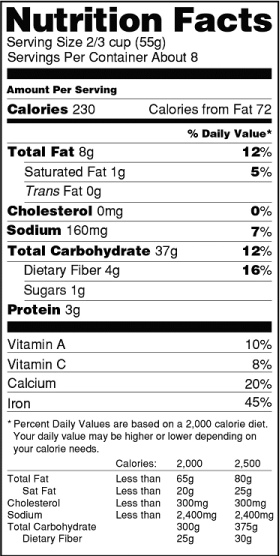
Current
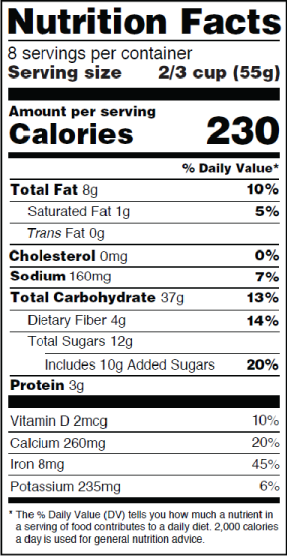
New
Get Expert Assistance
Registrar Corp’s Regulatory Specialists can update your label for compliance with FDA’s new rules.
Why You Should Start Now
Compliance with these rules may take considerable time. FDA extended the previous compliance date over industry concern for meeting the original deadline:
"Companies and trade associations with members covered by the rules have informed us that they have significant concerns about their ability to update all their labels by the compliance dates due to issues regarding (among other things) the need for upgrades to labeling software, the need to obtain nutrition information from suppliers, the number of products that would need new labels, and a limited time for reformulation of products." (82 FR 45753)
As the compliance deadline nears, testing laboratories may see a higher volume of food manufacturers requesting tests to account for values of newly required nutrients such as vitamin D and potassium. High demand may result in an increase of testing costs as well as potential scheduling difficulties and delays as laboratories near capacity.

What Changes on January 1?
Below are just a few examples of the extensive changes to the Nutrition Facts label:
-
Type Size for Certain Elements
Larger, bolded type for “Calories” and “Serving Size” declarations.
-
New Declaration for “Added Sugars”
Sugars added during processing, sugars intended to be added to food, and certain naturally-occuring sugars must be declared separately from “Total Sugars.”
-
New Footnote
A truncated footnote now defines % Daily Value (DV).
Foods intended for children aged 1 through 3 must specify “1,000 calories a day is used for general nutrition advice.”

-
Updated Serving Sizes
New reference amounts customarily consumed (RACC) for certain product categories change serving sizes and daily values.
Products containing between 200 and 300 percent of their RACCs must display an additional column of nutrition information for the whole package.
-
Updated Nutrient Requirements
Quantities for vitamin D, potassium, calcium, and iron must be listed and expressed in milligrams or micrograms.
Registrar Corp Makes Compliance Easy
Registrar Corp’s Regulatory Specialists can update your label for compliance with FDA’s new food labeling rules.
Registrar Corp’s Labeling and Ingredient Review Service Includes:

A detailed report of recommended revisions to your label
A ready-to-use graphic file of your FDA-compliant food label

One-on-one expert assistance from a Regulatory Specialist

One additional revision of the same label within 30 days

