Get a Quote
Cost-effective compliance is only a few clicks away
Meet The FDA Compliance Monitor
Know Sooner. Act Faster. Reduce Risk.
Now you can track your suppliers’ compliance, gauge your suppliers’ risk, and manage your suppliers’ documentation from one simple and secure platform.
Supply-Chain Management
Track and approve your suppliers in one centralized interface.
Automated Supplier Checks
Get current information on your suppliers, aggregated from six FDA databases.
Email Alerts
Receive immediate alerts when a supplier’s compliance changes.
Topnotch Security
Protect your data by managing supplier compliance in a highly secure environment.
Continuously Monitor Suppliers For:

FDA Registration Numbers

FDA Inspection Classifications

Import Alerts

Warning Letters

Import Refusals

Recalls
Customer Testimonials
We were looking at a great deal of time and money needing to be spent to manually research and track over 150 suppliers in our importing business. After investigating several options, we felt that FDA Monitor from Registrar Corp was the perfect solution for our situation. The company understands the subtleties of the importing business, and the complexities of working with international suppliers in multiple countries.
– Steve Brunsting
Vendor Compliance & Sourcing Manager | Limson Trading Inc.
A top feature is the ability to link the document system with the existing multi-supplier monitoring. Another important feature of the DMS is the rapid response of Registrar Corp’s development team that allows for customization and constant improvement. The customer service response times to our suggestions have been excellent.
– Frederick Robinson
Director of R&D and Co-pack Quality | Jasper Wyman & Son
Dive Deeper into Compliance with

A powerful scoring system that helps evaluate the risk your suppliers may present based on their FDA compliance and shipment history.

Evaluate supplier risk using a score of 0 to 100
View factors that impact a supplier’s score
Assess the risk of your supplier’s individual products
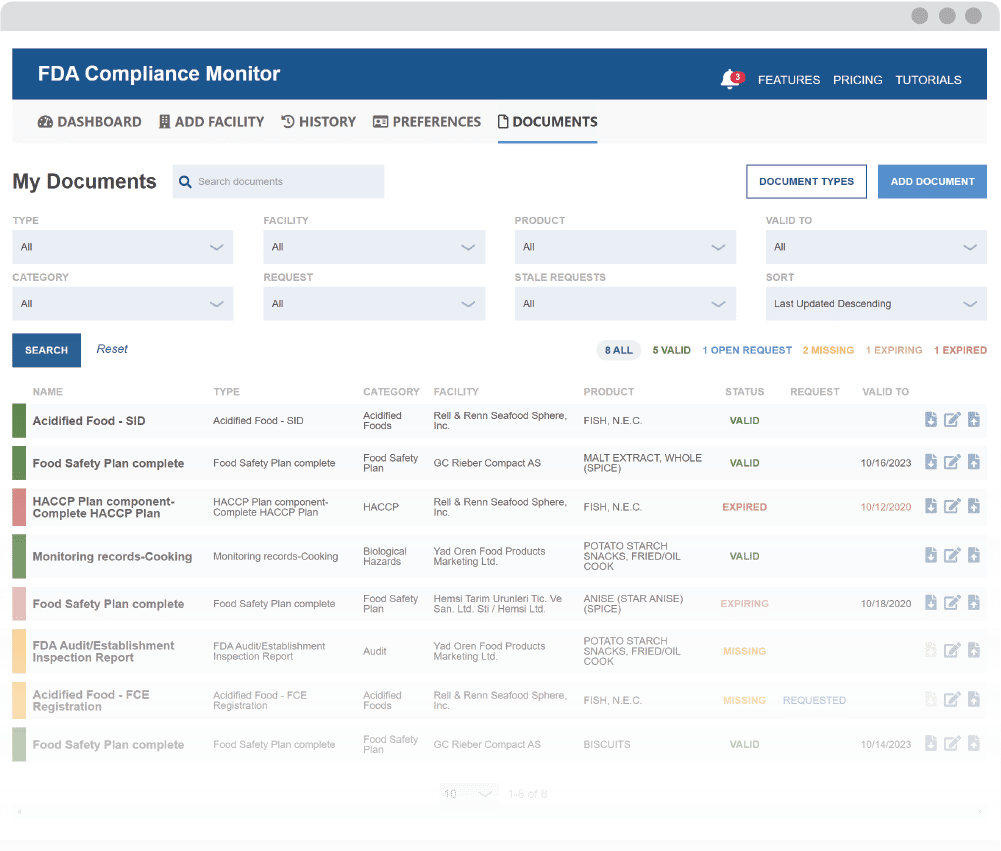
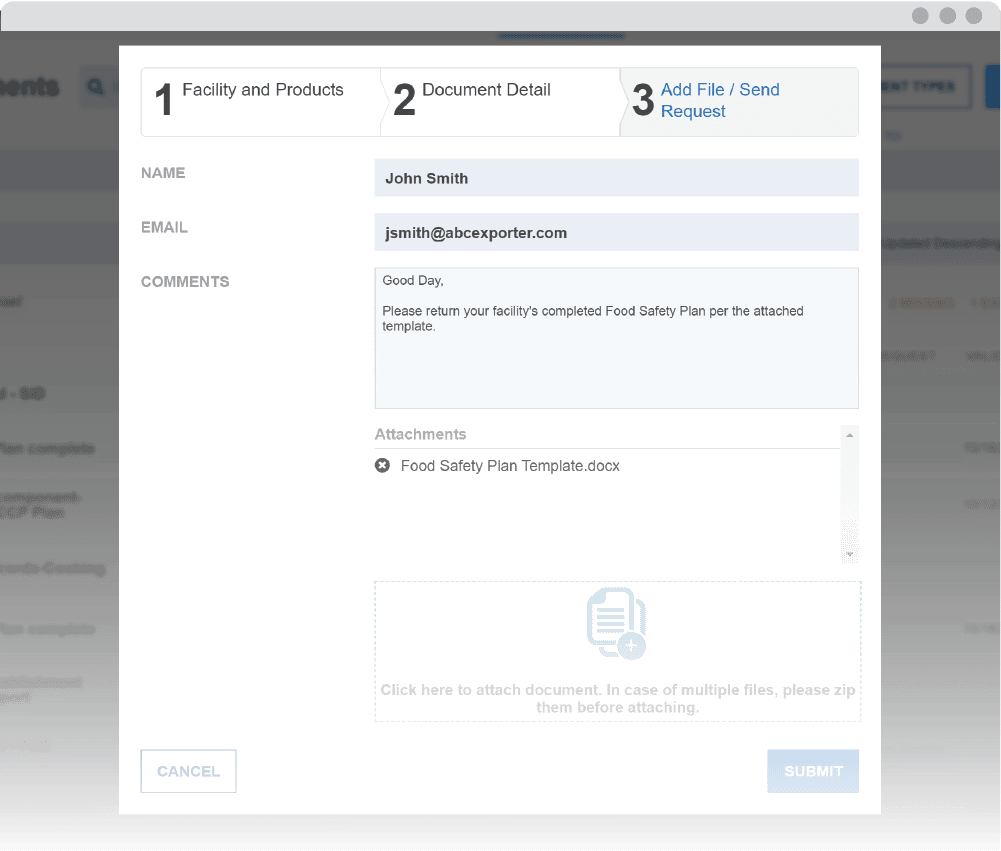
Enhance your monitoring with the Document Management System (DMS)
Maintain Required Recordkeeping and Supplier Documentation

 Receive automated actionable notifications
Receive automated actionable notifications
The Monitor’s notification system sends alerts when documents are ready to review and when they need to be re-assessed. Easily maintain compliance with recordkeeping requirements in spite of challenges, such as tracking document validity and staffing changes.
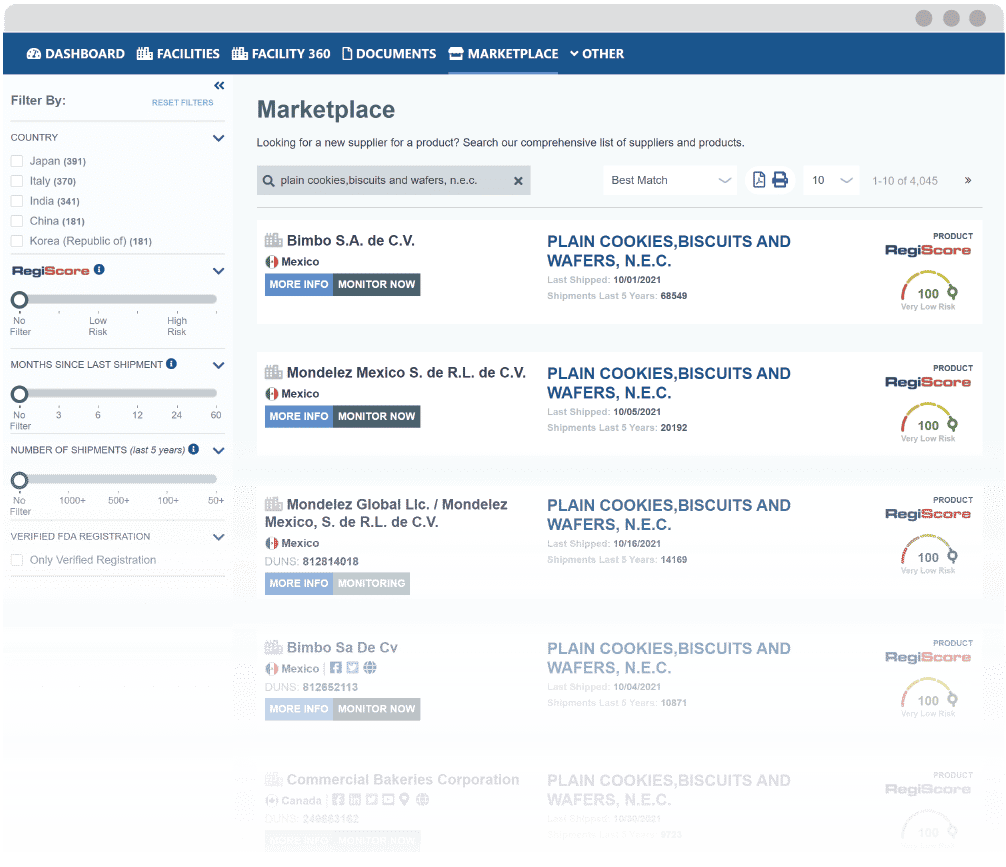
Transform Your Supplier Discovery with Marketplace
-
Source global suppliers suppliers with proven track records of consistent FDA compliance on a single platform.
-
Narrow potential suppliers by factors such as RegiScore, shipment volume and frequency, or exporting country.
-
Add potential suppliers to your account to continuously monitor their FDA compliance and manage their documentation.
Supplier Monitoring Is Required Under FSMA
FDA FSVP Rule
Deadlines Passed March 19, 2018
FDA’s Foreign Supplier Verification Program (FSVP) rule requires food importers to conduct and document an evaluation of their foreign suppliers’ FDA compliance status, “including whether the foreign supplier is the subject of an FDA warning letter, import alert, or other FDA compliance action related to food safety.” This requirement cannot be fulfilled by your customs broker.
FDA Preventive Controls Rule
Deadlines Passed September 18, 2017
FDA’s Preventive Controls Rules require registered food facilities to establish, implement and document a supply-chain program monitoring their suppliers’ FDA compliance status, “including an FDA warning letter or import alert relating to the safety of food and other FDA compliance actions…”