Introducing The Only Complete FSVP Documentation Solution
Registrar Corp’s Document Management System (DMS), powered by the FDA Compliance Monitor, is purpose-built to request, approve, and maintain critical food safety documents required under the Foreign Supplier Verification Program (FSVP) Rule.
Never rely on spreadsheets, email chains, or local file systems again. The DMS is your simple, secure solution to FSVP documentation requirements.
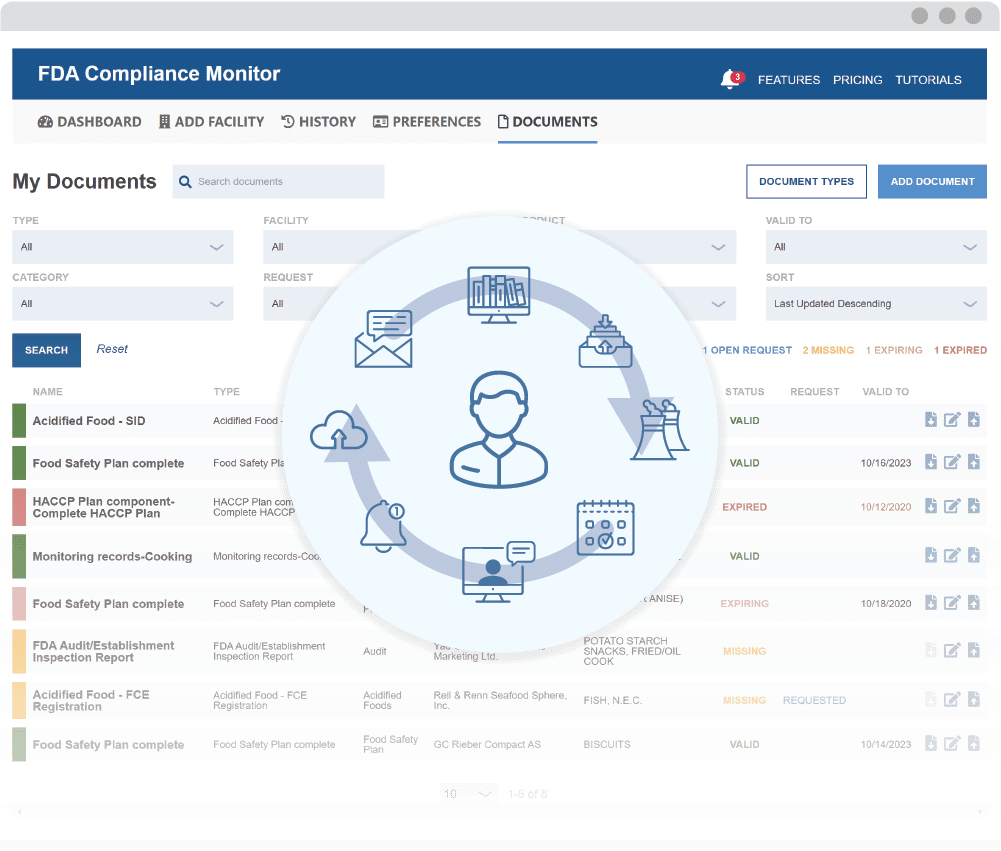
The Proactive Approach to FSVP Compliance
Combine Automated Supplier Monitoring with Innovative Document Management
Automated Expiration Alerts
Get notified and automatically re-request documents when re-evaluation deadlines are approaching.

Centralize Critical Documents
Organize and filter files to quickly identify actionable items.

Robust Template Library
Use preset document types or create your own with custom attachments.

Always Audit Ready
Access files anytime, anywhere from a cloud-based platform.

Secure Supplier Data
Data is protected using end-to-end encryption, backup systems, and role-based access control.

Comply with FSVP
Designed to help satisfy FDA supplier monitoring and record-keeping requirements.
Experience the Full Capability of the Leading FSVP Compliance Solution
The FDA Compliance Monitor’s comprehensive suite of tools helps prepare you for an FSVP Inspection
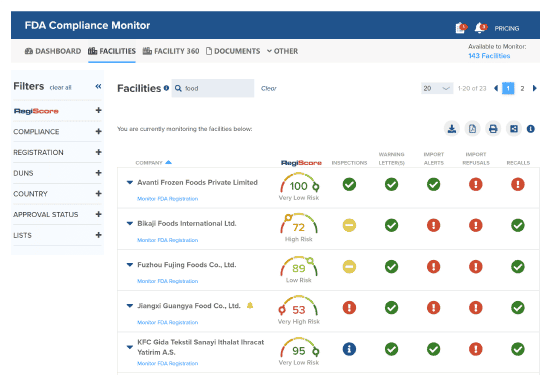
Track Supplier Compliance
Monitor and approve suppliers in one centralized interface. Get alerts when suppliers are subject to recalls, Warning Letters, or other FDA enforcement.
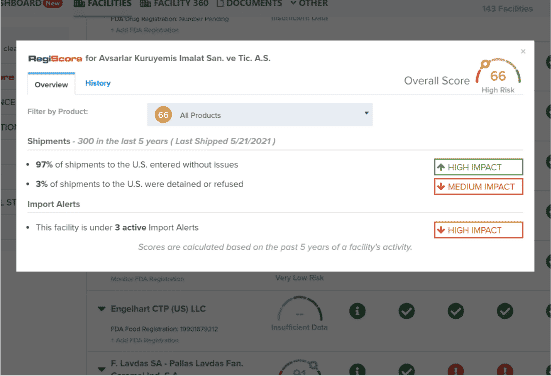
Identify Supplier Risk
Patent-pending RegiScore technology uses predictive analytics to calculate the potential risk of importing from a supplier.
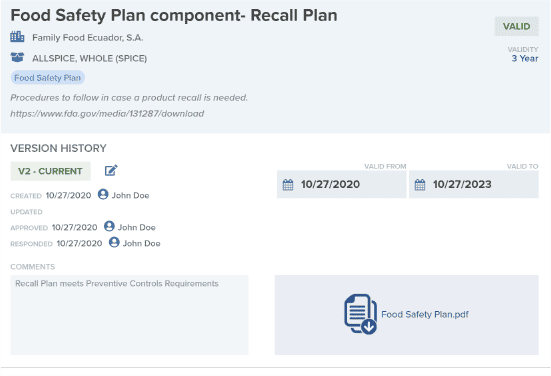
Manage Supplier Documentation
Request, evaluate, and approve your suppliers’ Food Safety Plans, Product Labels, Certifications, and more.
The Document Management System (DMS) is a timely solution to an increasingly difficult issue of following up on supplier document submissions as our supplier base grows. The new system will replace our internal filing and database management system for supplier follow-ups.
– Frederick Robinson
Director of R&D and Co-pack Quality | Jasper Wyman & Son
Frequently Asked Questions
How does Registrar Corp’s DMS help me comply with FSVP?
Under the Foreign Supplier Verification Program (FSVP) rule, FDA requires U.S. Importers to document their evaluation and approval for each product imported from their foreign suppliers. Many Importers choose to request and evaluate suppliers’ Food Safety Plans, audit reports, and GFSI certifications to fulfill this requirement.
FDA document control, collection, storage, and re-evaluation is a challenging long-term problem, especially considering factors such as navigating document versions, maintaining supplier contact lists, and adapting to in-house staffing changes. Registrar Corp’s DMS is the leading solution to all of these and more.
What types of documents can I manage?
The DMS allows you to manage any document you need including Food Safety Plans, GFSI Certifications, Inspection Reports, Process Documentation, Product Labels, and more. Use one of Registrar Corp’s premade document types to categorize your documents or create your own with custom attachments.
How can Registrar Corp’s DMS save me time and money?
Without Registrar Corp’s DMS, it takes an estimated minimum of 2 hours per supplier per month at an average of $24 per hour for a qualified individual to manage document collection, tracking, review, and approval.
The DMS removes the tedium of navigating email chains, writing reminders, and checking calendars to maintain FSVP documentation. When documents near expiration, the DMS will send email reminders to reassess and can even automatically request updated documents from your suppliers.
Combining document management with supplier monitoring, the full FDA Compliance Monitor suite can save you as much as $12,000 per year with as few as 5 suppliers.
Can documents reaching expiration be automatically requested by the system?
Yes. You can effortlessly re-request supplier documents by setting a document to “auto-renew.” When an auto-renewed document approaches expiration, the DMS will request updated documents from your supplier without any additional clicks.
How many documents can I store?
You can store as many documents as you need. The DMS does not place any limits on your documents counts, versions, sizes, or types. All of your food safety documents are seamlessly retrievable from an easy-to-use interface.
Will Registrar Corp help me obtain documents from my suppliers?
Yes. Your dedicated account manager will ensure smooth implementation of your entire account, including onboarding your suppliers and requesting documents from them on your behalf.
How many users can I have?
Unlike other solutions, Registrar Corp doesn’t charge more for additional users. Easily add your entire team to the DMS with unlimited seats on your account.
How is this solution so comprehensive?
Registrar Corp’s DMS was designed with the help of food scientists implementing FSVPs on a daily basis. Their insight on FSVP best practice has allowed us to create a continuously improving, adaptive solution that makes compliance easy and affordable.
How affordable is this solution?
Registrar Corp’s full FDA Compliance Monitor suite helps you fulfill FSVP requirements for less than 1/4 the cost of maintaining supplier evaluation systems in house.

