資料
インフォグラフィック
推奨資料

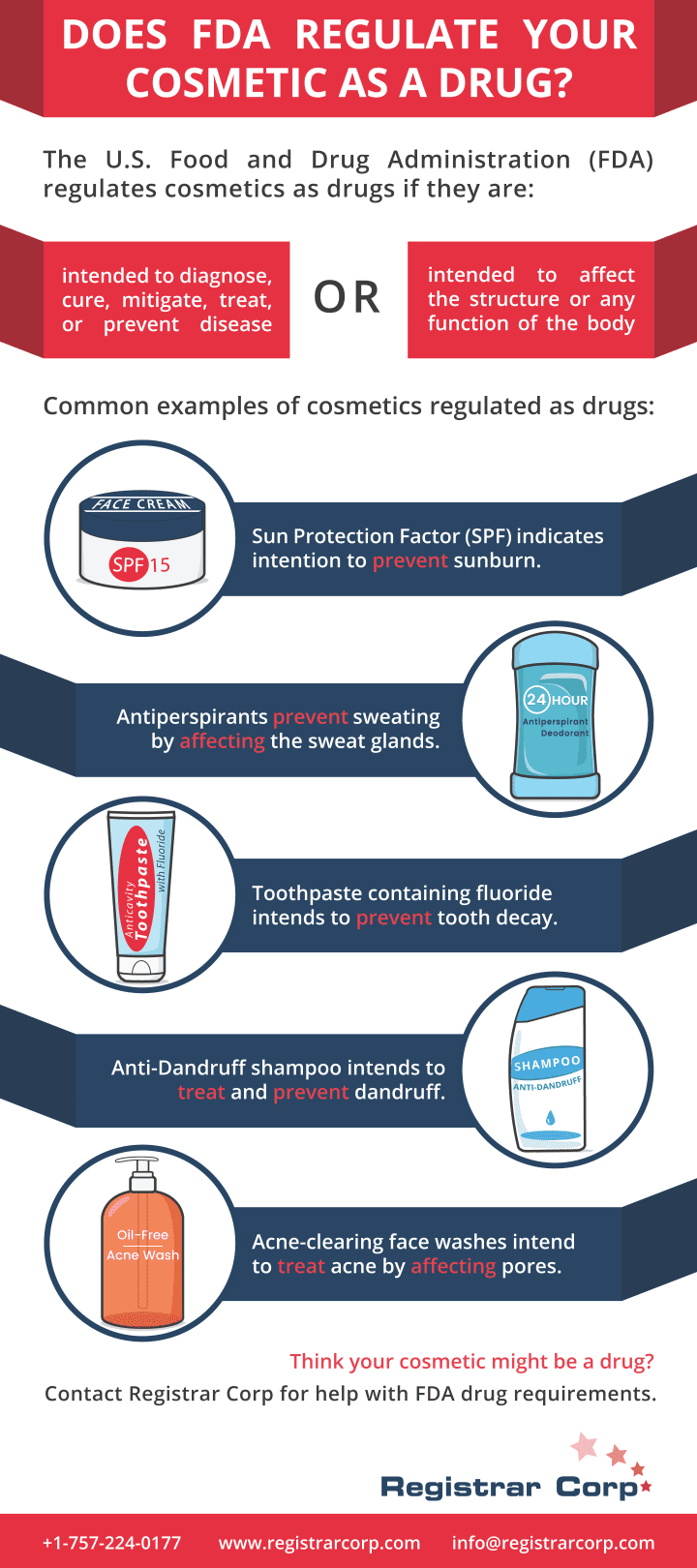
FDAの承認を得る方法
すべてのFDA規制製品が米国で販売される前にFDAの承認を必要とするいうのははよくある誤解です。レジストラ・コープは、製品がFDAの承認を必要とするかどうかの判断のためにこのガイドを作成しました。

FDAとUSDA –誰が何を規制するか
肉製品または家禽製品の規制主体がFDAなのかUSDAなのかを判断するのは難しい場合があります。 レジストラ・コープは、FDAとUSDAの管轄の違いを明確にするのに役立つガイドを作成しました。


ビデオ
米国FDA規制に対応する支援
米国FDA食品業者登録
FDA Compliance Monitor
Facility 360
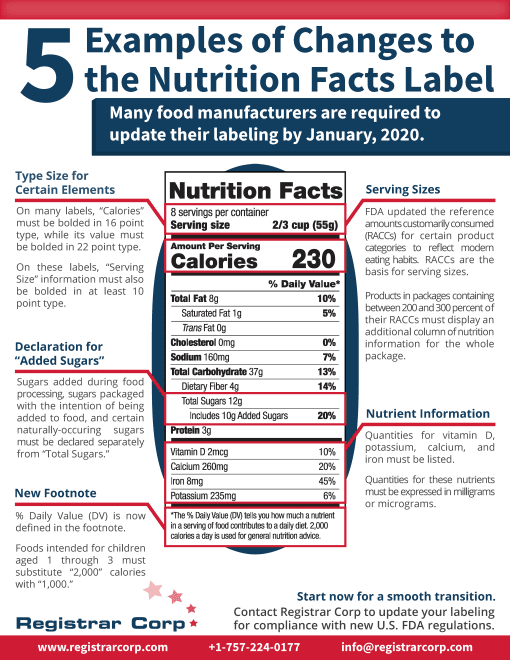
米国FDA食品表示規制
米国FDA FCE-SID規制
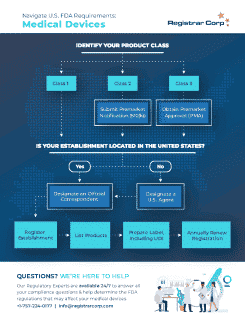
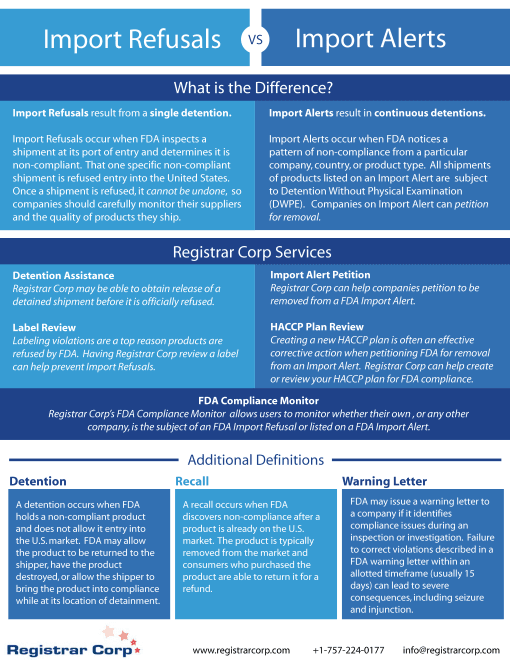
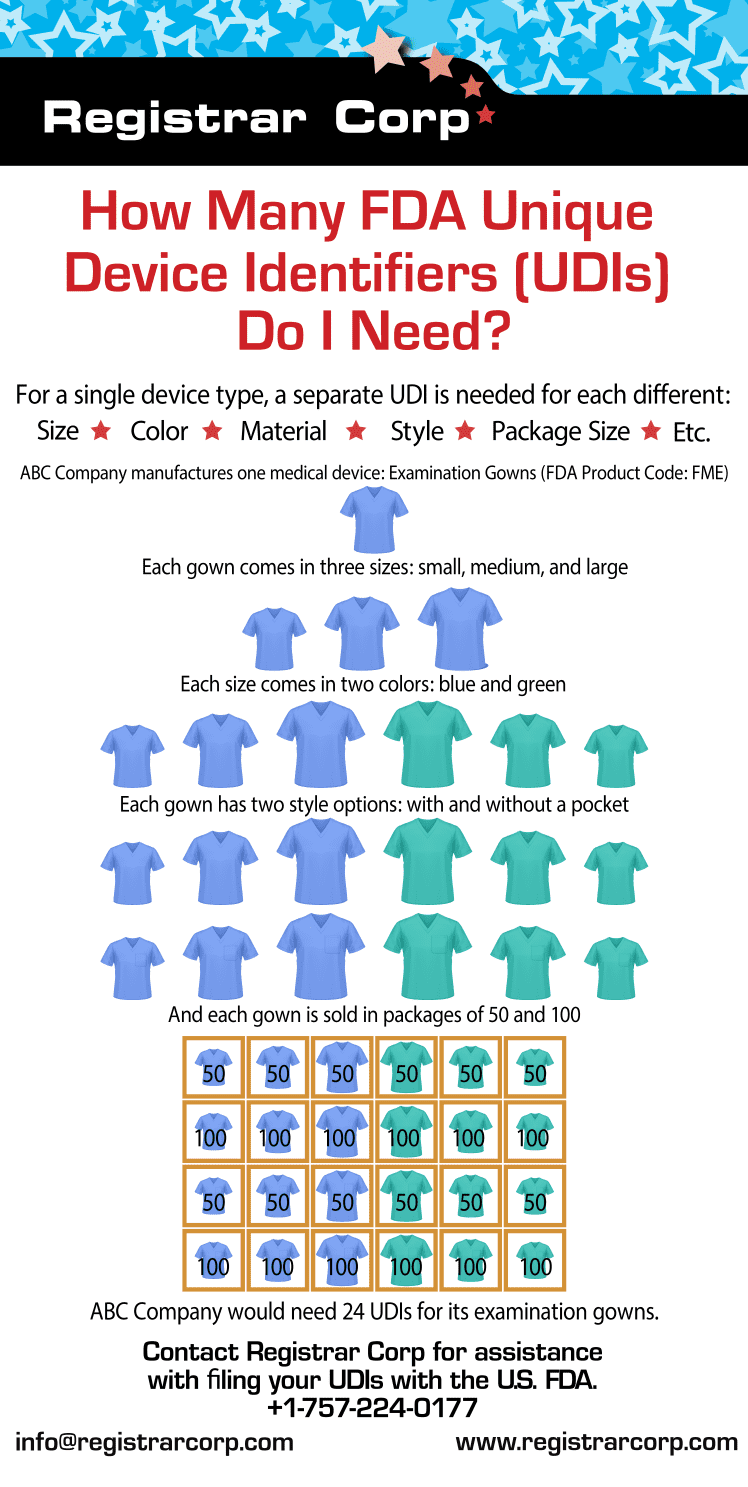
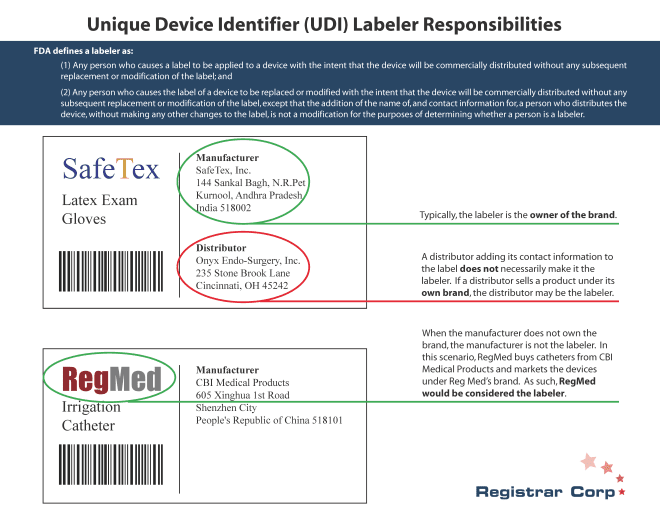
米国FDA 特定デバイス特定識別子(Unique Device Identifier)(UDI)
コンプライアンスツール
FDA Compliance Monitor Savings Calculator
Registrar Corp’s FDA Compliance Monitor saves you thousands of dollars on your supplier monitoring process. See how much today.
Get StartedCheck your RegiScore
U.S. Importers use RegiScore to gauge the risk of buying from your company. Check your RegiScore for free.
Get StartedQualified Facility Attestation Wizard
Registrar Corp’s Qualified Facility Wizard can help determine whether your facility is eligible to file a Qualified Facility Attestation.
Get StartedFCE Wizard
Registrar Corp’s FCE Wizard is a free tool that allows users to identify whether their products are subject to FCE and SID regulations.
Get StartedSID Verifier
Registrar Corp’s SID Verifier is a free tool that allows users to verify whether SIDs are currently on file in FDA’s database.
Get StartedAdditional Tools
Prior Notice Express
Registrar Corp’s Prior Notice Express makes filing prior notice quick and easy and decreases potential filing errors.
Get StartedFDA Compliance Monitor
Registrar Corp’s FDA Compliance Monitor® allows users to easily monitor the compliance status and history of any FDA-regulated company.
Get StartedFacility 360
Track your compliance, gauge your risk to U.S. Importers, and monitor your shipments all in one simple, secure platform.
Get StartedShipment Monitoring
Protect Your Company From Unauthorized Shipments. Monitor shipments associated with your FDA Registration Number.
Get StartedWebinars
-

Compliance Requirements for U S Food and Drug Administration FDA by Registrar Corp
-

U.S. FDA Regulations for Animal Feed
-

Six Factors that Affect Your Shipments to the U.S.
-

How to Prepare for an FDA Inspection
-

PCQI 101: How to Become a Preventive Controls Qualified Individual and Why
-

U.S. FDA Foreign Supplier Verification Program (FSVP) Requirements
-

U.S. FDA Preventive Controls Requirements
-

U.S. FDA Food Labeling Rules - The New Normal
-

3 Ways to Protect Your Brand with Facility 360
-

PCQI 101: How to Become a Preventive Controls Qualified Individual and Why
-

GFSI Certification 101
-

FDA Registration and U.S. Labeling Requirements for Winemakers