Transform Your Supplier Discovery
Registrar Corp’s Marketplace is the only supplier discovery platform that incorporates historic FDA compliance and shipment data for every supplier’s product. This comprehensive database provides the complete compliance history of products from more than 500,000 different suppliers.
Now you can be assured of finding new suppliers with a long record of shipping compliant products.
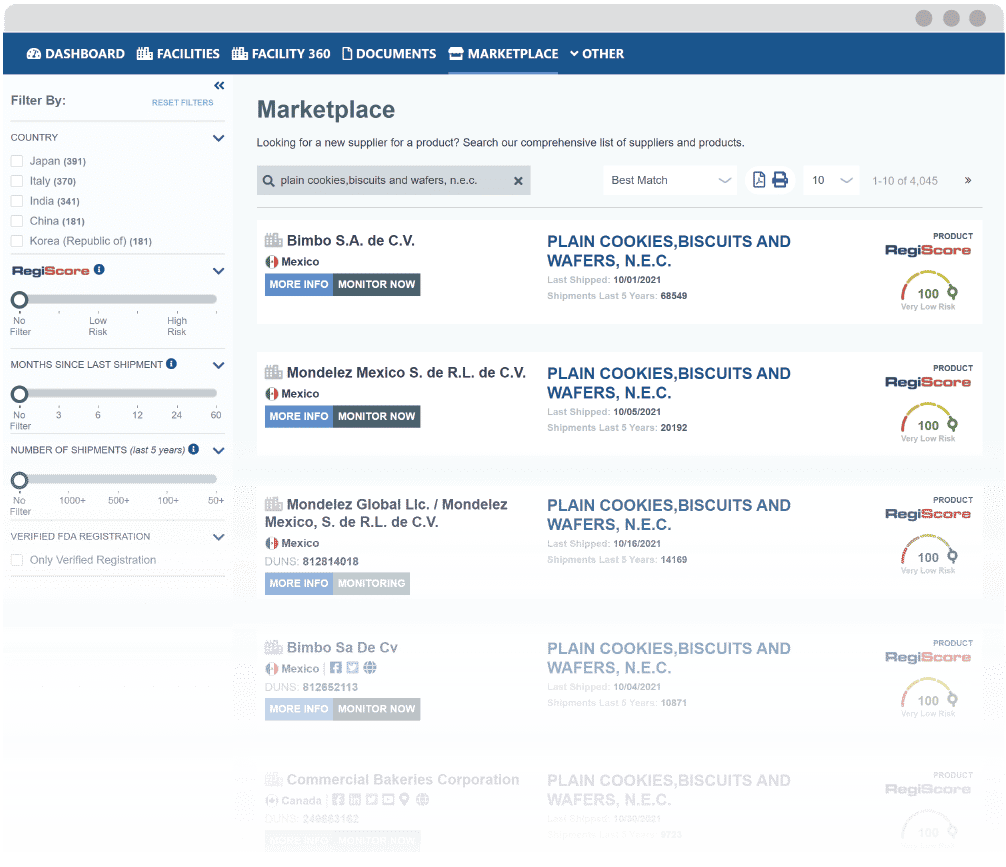
Find New Suppliers In Minutes
Source Compliant Suppliers with Actionable Shipment Data

ACCESS 100% OF SUPPLIERS
Access every supplier that shipped a FDA-regulated product to the U.S. in the past 5 years. Other discovery platforms are opt-in, offering limited and unverified supplier data.

QUALITY ASSURANCE
Marketplace makes historic FDA compliance data transparent to buyers. Now it’s easy to assess supplier risk before you purchase.

SUPPLY CHAIN CERTAINTY
Provides product shipment data that assures consistent supply as well as easy supplier replacement.
Experience the Full Capability of the Leading FSVP Compliance Solution
The FDA Compliance Monitor’s comprehensive suite of tools helps prepare you for an FSVP Inspection
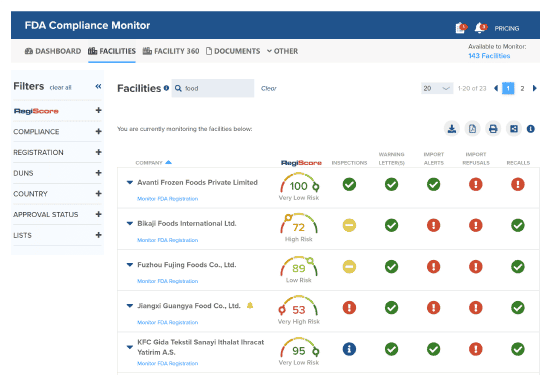
Track Supplier Compliance
Monitor and approve suppliers in one centralized interface. Get alerts when suppliers are subject to recalls, Warning Letters, or other FDA enforcement.
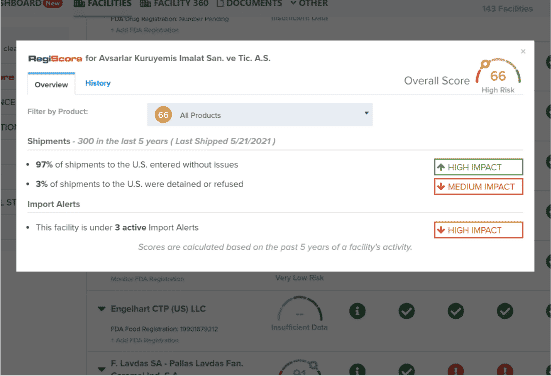
Identify Supplier Risk
Patent-pending RegiScore technology uses predictive analytics to calculate the potential risk of importing from a supplier.
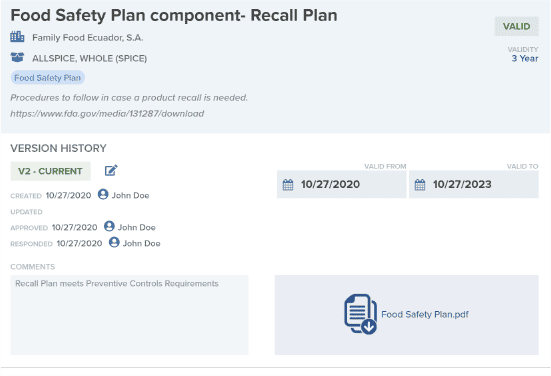
Manage Supplier Documentation
Request, evaluate, and approve your suppliers’ Food Safety Plans, Product Labels, Certifications, and more.
Get a Quote
Compliance-enhanced supplier discovery is only a few clicks away

