Automate Supply Chain Risk Management
Easily identify vulnerabilities in your supply chain using the FDA Compliance Monitor.
NOW FEATURING: BUYER INSIGHTS
Leverage this unrivaled technology and never-before-seen supplier
data to streamline and diversify your supply chain easier than ever before.
Registrar Corp assists businesses with U.S. FDA compliance.
Registrar Corp is not affiliated with the U.S. FDA
Streamline your supply chain management today.
Cost-effective compliance is only a few clicks away
Meet The FDA Compliance Monitor
Know Sooner. Act Faster. Reduce Risk.
Now you can track your suppliers’ compliance, gauge your suppliers’ risk, and manage your suppliers’ documentation from one simple and secure platform.
Supply-Chain Management
Track and approve your suppliers in one centralized interface.
Automated Supplier Checks
Get current information on your suppliers, aggregated from six FDA databases.
Email Alerts
Receive immediate alerts when a supplier’s compliance changes.
Topnotch Security
Protect your data by managing supplier compliance in a highly secure environment.
Continuously Monitor Suppliers For:

FDA Registration Numbers

FDA Inspection Classifications

Import Alerts

Warning Letters

Import Refusals

Recalls
Customer Success Stories
Customer Testimonials
We were looking at a great deal of time and money needing to be spent to manually research and track over 150 suppliers in our importing business. After investigating several options, we felt that FDA Monitor from Registrar Corp was the perfect solution for our situation. The company understands the subtleties of the importing business, and the complexities of working with international suppliers in multiple countries.
– Steve Brunsting
Vendor Compliance & Sourcing Manager | Limson Trading Inc.
A top feature is the ability to link the document system with the existing multi-supplier monitoring. Another important feature of the DMS is the rapid response of Registrar Corp’s development team that allows for customization and constant improvement. The customer service response times to our suggestions have been excellent.
– Frederick Robinson
Director of R&D and Co-pack Quality | Jasper Wyman & Son
Case Studies

Registrar Corp software and tech-enabled services help wholesale importer save more than $50,000 annually

Registrar Corp saves small business $16,000+ annually through tech-enabled services and software solutions
Dive Deeper into Compliance with

A powerful scoring system that helps evaluate the risk your suppliers may present based on their FDA compliance and shipment history.

Evaluate supplier risk using a score of 0 to 100
View factors that impact a supplier’s score
Assess the risk of your supplier’s individual products
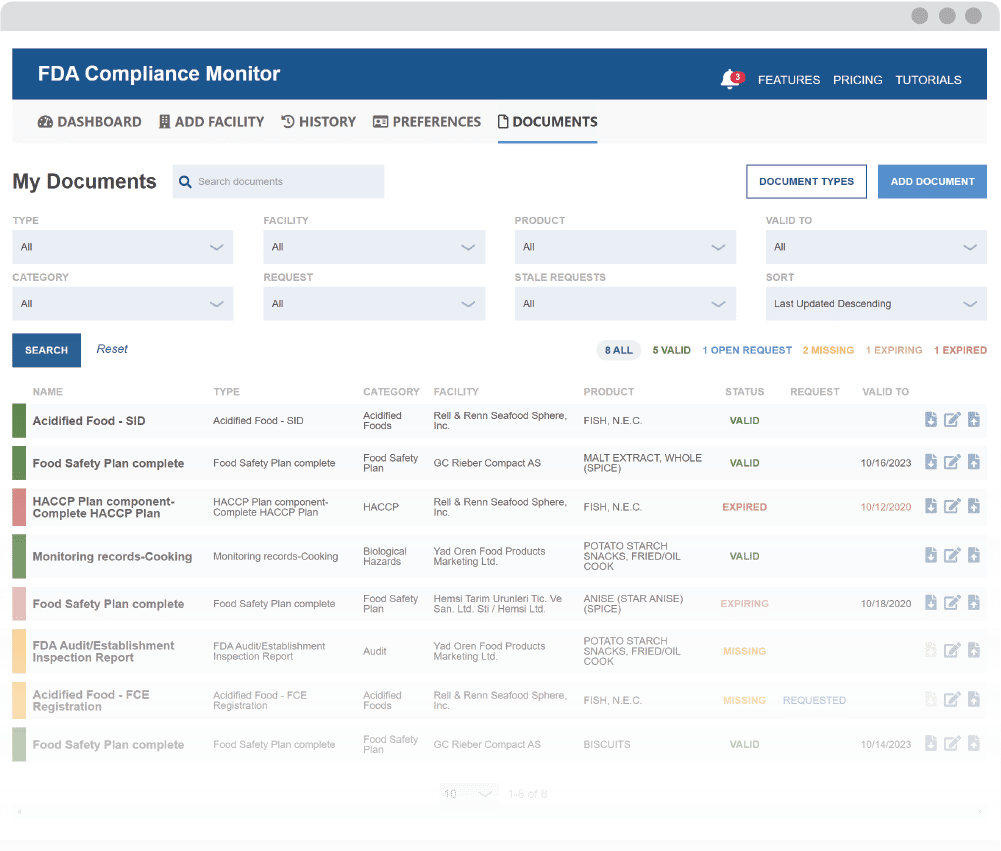
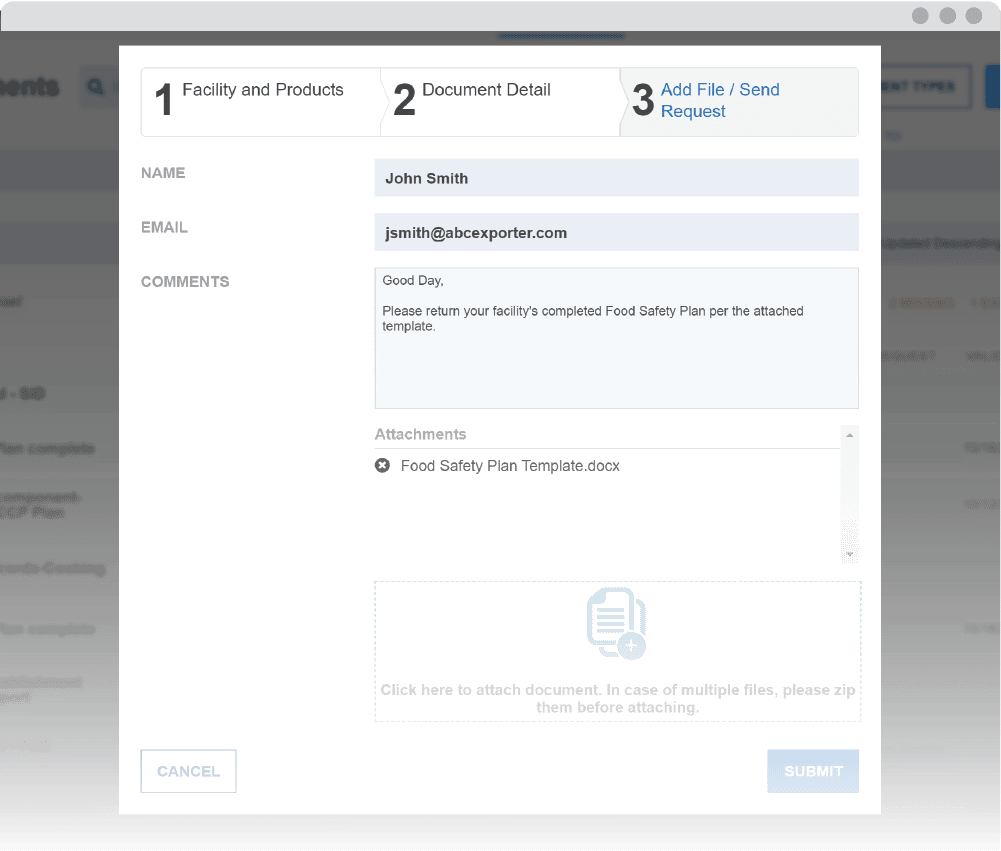
Enhance your monitoring with the Document Management System (DMS)
Maintain Required Recordkeeping and Supplier Documentation

 Receive automated actionable notifications
Receive automated actionable notifications
The Monitor’s notification system sends alerts when documents are ready to review and when they need to be re-assessed. Easily maintain compliance with recordkeeping requirements in spite of challenges, such as tracking document validity and staffing changes.
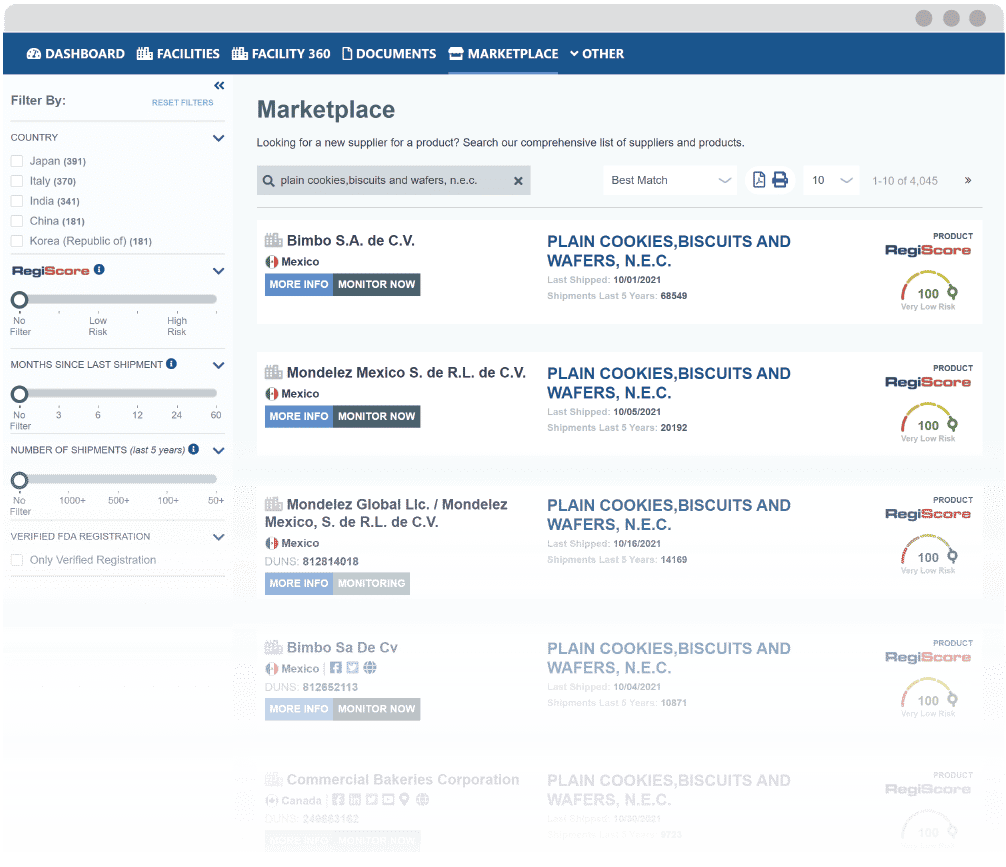
Transform Your Supplier Discovery with Marketplace
-
Source global suppliers with proven track records of consistent FDA compliance on a single platform.
-
Narrow potential suppliers by factors such as RegiScore, shipment volume and frequency, or exporting country.
-
Add potential suppliers to your account to continuously monitor their FDA compliance and manage their documentation.
Instantly Connect With US Importers Through Exporter Marketplace
-
Upload photos to help your products stand out
-
Return in search results with priority
-
Connect with buyers that are searching for products you provide
-
Quickly send importers certifications and documentation that demonstrate your facility’s compliance with FDA regulations

Protect Your Company from Unauthorized Shipments
-
Analyze trends in your shipments by product and by compliance status.
-
Easily pinpoint products that delay your shipments so that you can implement corrective actions and minimize future detentions.
-
Find a list of each shipment by line, including pertinent details that will help you identify suspicious shipments associated with your registration number.

PERSONALIZE YOUR SUPPY-CHAIN COMPLIANCE
Automated Compliance Cost-Saving Packages
Essentials
Basic tools to simplify
compliance
Starting at $1,595
 Compliance Monitor Compliance Monitor |
 Document Management System Document Management System |
 In-app notifications and real-time alerts In-app notifications and real-time alerts |
 RegiScore: A proprietary risk score calculation tool RegiScore: A proprietary risk score calculation tool |
 Dedicated FSVP compliance support Dedicated FSVP compliance support |
| – |
| – |
| – |
| – |
| – |
| – |
Professional
Essentials package +
Extensive risk aversion and automation
Starting at $3,995
 Compliance Monitor Compliance Monitor |
 Document Management System Document Management System |
 In-app notifications and real-time alerts In-app notifications and real-time alerts |
 RegiScore: A proprietary risk score calculation tool RegiScore: A proprietary risk score calculation tool |
 Dedicated FSVP compliance support Dedicated FSVP compliance support |
 Marketplace Marketplace |
 Buyer Insights Buyer Insights |
 Automated Supplier Feed Automated Supplier Feed |
 SmartDMS SmartDMS |
| – |
| – |
Complete
Professional package +
FSVP management by Registrar Corp’s Food Safety Specialists
Starting at $9,995
 Compliance Monitor Compliance Monitor |
 Document Management System Document Management System |
 In-app notifications and real-time alerts In-app notifications and real-time alerts |
 RegiScore: A proprietary risk score calculation tool RegiScore: A proprietary risk score calculation tool |
 Dedicated FSVP compliance support Dedicated FSVP compliance support |
 Marketplace Marketplace |
 Buyer Insights Buyer Insights |
 Automated Supplier Feed Automated Supplier Feed |
 SmartDMS SmartDMS |
 Writing/Reviewing FSVP plans Writing/Reviewing FSVP plans |
 FSVP Implementation FSVP Implementation |